Researchers from the Nanomol Group initiate contacts with the Spanish Agency for Medicines and Health Products (AEMPS) to bring a new treatment against venous leg ulcers to clinical practice
Researchers of the Nanomol group of the Institute of Materials Science of Barcelona (ICMAB-CSIC), together with the company Nanomol Technologies S.L. have begun a consultation process with the Spanish Association of Medicines and Health Products (AEMPS) to try and enter a new treatment in a clinical studies stage, to fight against venous ulcers in lower extremities. The study is carried out within the framework of the RIS3CAT NANONAFRES project.
Approximately, 80 % of ulcers in lower extremities have associated pathologies of venous insufficiency and these wounds are usually recurrent. An open ulcer can take from weeks to years to close, increasing the risk of mortality and affecting the quality of life of patients who suffer from them, mostly elderly people. The solution proposed within the NANONAFRES project consists of a medicine for topical application based on the incorporation of a biomolecule, with skin regenerating activity, encapsulated in nanoparticles. This approach ensures the protection of the active substance, preventing its degradation, while allowing the controlled and direct release of the drug in the area of the ulcer.
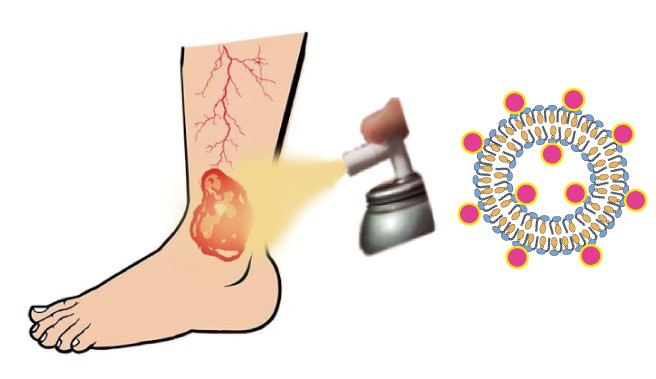
Figure: Scheme of the proposed methodology, based on nanoparticles, for the treatment of venous leg ulcers. / Nanomol Group, ICMAB-CSIC
The NANONAFRES project, led by Nanomol Technologies SL, was born from the collaboration of the Nanomol group from ICMAB-CSICwith the companies Nanomol Technologies and BIOMED-LEITAT, the primary care centres EAP Osona Sur-Alt Congost SLP and the EAP Vallcarca-Sant Gervasi, public hospitals such as the Terrassa Health Consortium and the Maresme Health-Consortium Foundation and international research centres such as the Centro de Ingeniería Genética y Biotecnología de Cuba. This project, awarded with a RIS3CAT fund sponsored by the Generalitat of Catalonia, has a budget of 2.3 million euros and a duration of 3 and a half years.
This first consulting meeting with the AEMPS is a very important milestone in the process of developing any drug, in order to carry out clinical trials and transfer the system to the market, and thus bring the proposed treatment to the people who need it.
This approach will improve the effectiveness of current venous ulcers treatments, as well as the quality of life of patients, reducing the cost of medicines available today. In addition, the Nanomol group already has an internationally granted patent on this product, which demonstrates the novelty of this system and strengthens its position to reach the market through the partners present in this consortium and, in particular, through its spin-off Nanomol Technologies S.L.
Links of interest:
- Article published on ICMAB’s site (2016): http://icmab.es/nanomol-participa-al-projecte-nanonafres-de-la-comunitat-ris3cat-de-nous-dispositius-de-diagnostic-i-big-data-aplicades-a-la-salut
- Article published on LEITAT’s site (2016): https://projects.leitat.org/nanonafres/
- Patent: “VESICLES COMPRISING EPIDERMAL GROWTH FACTOR AND COMPOSITIONS THEREOF” (WO2014019555): https://patents.google.com/patent/US9717776
- Download the PRESS RELEASE in CATALAN, SPANISH and ENGLISH.






